Adenosine Triphosphate and ATP Hydrolysis
Adenosine triphosphate (ATP) is a nucleoside triphosphate molecule used in cells as a coenzyme which is essentially 'coupled' with a reaction in the body. Coupled reactions can be defined as reactions where the product of one reaction (Rxn A) is consumed as reactant for another reaction (Rxn B). The reaction slightly producing product (Rxn A) has a smaller K value (K = the equilibrium constant. This is when there is no net change between reactants and products. The forward and reverse reactions occur at equal rates, so adding more reactant or product will no longer effect the reaction, i.e K = A + B  C, where K = a value that represents the concentration of product vs. the concentration of reactants at equilibrium, K = [C]^c / [A]^a [B]^b) compared to the reaction (Rxn B) that consumes it. If K value is large, then the concentration (at equilibrium) of C is greater than the concentration of A times B (at equilibrium). This means that the reaction is favorable towards the product, since more product is formed compared to reactants. An example of a reaction with a small K value is when glutamate is combined with ammonia to produce glutamine. Glutamine is an amino acid responsible for acid-base regulation in the kidney by producing ammonium (an acidic compound), cellular energy, nontoxic transporter of ammonia through the blood and much more. This reaction on its own has a K value of 8e10^-6, meaning that if the reaction happens on its own, not enough glutamine will be produced for the body. This is where ATP is coupled with the reaction to help produce glutamine at a greater rate. The ATP reaction occurs this way: ATP + H2O → ADP+ Pi, where K is a lot greater than 1. This reaction is called ATP hydrolysis.
C, where K = a value that represents the concentration of product vs. the concentration of reactants at equilibrium, K = [C]^c / [A]^a [B]^b) compared to the reaction (Rxn B) that consumes it. If K value is large, then the concentration (at equilibrium) of C is greater than the concentration of A times B (at equilibrium). This means that the reaction is favorable towards the product, since more product is formed compared to reactants. An example of a reaction with a small K value is when glutamate is combined with ammonia to produce glutamine. Glutamine is an amino acid responsible for acid-base regulation in the kidney by producing ammonium (an acidic compound), cellular energy, nontoxic transporter of ammonia through the blood and much more. This reaction on its own has a K value of 8e10^-6, meaning that if the reaction happens on its own, not enough glutamine will be produced for the body. This is where ATP is coupled with the reaction to help produce glutamine at a greater rate. The ATP reaction occurs this way: ATP + H2O → ADP+ Pi, where K is a lot greater than 1. This reaction is called ATP hydrolysis.
 C, where K = a value that represents the concentration of product vs. the concentration of reactants at equilibrium, K = [C]^c / [A]^a [B]^b) compared to the reaction (Rxn B) that consumes it. If K value is large, then the concentration (at equilibrium) of C is greater than the concentration of A times B (at equilibrium). This means that the reaction is favorable towards the product, since more product is formed compared to reactants. An example of a reaction with a small K value is when glutamate is combined with ammonia to produce glutamine. Glutamine is an amino acid responsible for acid-base regulation in the kidney by producing ammonium (an acidic compound), cellular energy, nontoxic transporter of ammonia through the blood and much more. This reaction on its own has a K value of 8e10^-6, meaning that if the reaction happens on its own, not enough glutamine will be produced for the body. This is where ATP is coupled with the reaction to help produce glutamine at a greater rate. The ATP reaction occurs this way: ATP + H2O → ADP+ Pi, where K is a lot greater than 1. This reaction is called ATP hydrolysis.
C, where K = a value that represents the concentration of product vs. the concentration of reactants at equilibrium, K = [C]^c / [A]^a [B]^b) compared to the reaction (Rxn B) that consumes it. If K value is large, then the concentration (at equilibrium) of C is greater than the concentration of A times B (at equilibrium). This means that the reaction is favorable towards the product, since more product is formed compared to reactants. An example of a reaction with a small K value is when glutamate is combined with ammonia to produce glutamine. Glutamine is an amino acid responsible for acid-base regulation in the kidney by producing ammonium (an acidic compound), cellular energy, nontoxic transporter of ammonia through the blood and much more. This reaction on its own has a K value of 8e10^-6, meaning that if the reaction happens on its own, not enough glutamine will be produced for the body. This is where ATP is coupled with the reaction to help produce glutamine at a greater rate. The ATP reaction occurs this way: ATP + H2O → ADP+ Pi, where K is a lot greater than 1. This reaction is called ATP hydrolysis.
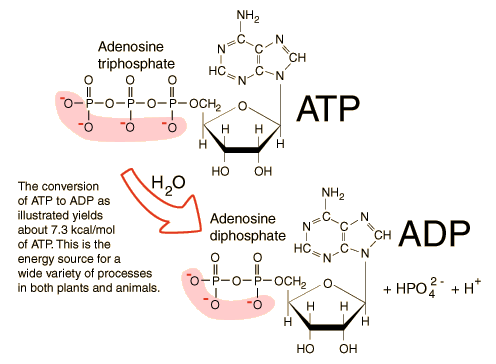
In this case, ATP hydrolysis is defined as a reaction in which chemical energy is released from the bonds between phosphate groups on the tail or the end of the ATP molecule.
 |
| Source: Animal Cell Biology |
Because the K value is large, the product is extremely favorable. The release of energy within the bonds, is used not only in these biochemical reactions, but also muscle contraction, metabolism: food and liquid break down, etc. ATP hydrolysis is used as a reaction coupled with an unfavorable (defined as a reaction that doesn't produce much product compared to reactants) reaction. The combination of the reactions is equal to adding both K values, giving an overall K value greater than one. This ensures that glutamine will be produced at a higher rate. K value > 1 is a product favored reaction at equilibrium. The hydrolysis of ATP is coupled with many reactions that would not naturally produce much product, especially at the rate in which ATP does when coupled with the specific reaction. Some examples of these products are oxidation/breakdown of ethanol (a highly toxic compound), muscle contraction to promote movement, energy for the sodium/potassium pump, and other metabolic reactions to keep organisms alive.
Gibbs 'Free Energy' and its Relation to ATP
At this point, I've associated K value to ATP. The larger the K value, the more product 'favored' the reaction, thus making the reaction move toward the products: a forward rate reaction. Because ATP hydrolysis has an overall K value greater than 1, it is coupled with 'unfavorable' reactions to produce product at a greater rate. The reason why ATP hydrolysis' equilibrium constant is greater than 1 is mostly due to the Gibbs 'free energy' constant (ΔG). ΔG tells us the directionality (spontaneity) of the reaction at any concentration. If ΔG < 0, the reaction is spontaneous (meaning it 'favors' the product), and if ΔG > 0 the reaction is considered non-spontaneous (reaction 'favors' reactants). If ΔG < 0, then K > 1, and if the G value > 0, then K < 1 and this due to thermodynamics.
Thermodynamics refers to the study of the effects of work, heat and energy and how it effects a system. A system is where the specific reaction takes place (where we want to focus on), and anything else around it is the surroundings. The system + the surroundings is defined as the universe. According to the Second Law of Thermodynamics, for a process to be spontaneous, the ΔS universe (S is a measure of how many ways a system can disperse energy at a given temperature. If we have a large number of microstates - 'motional freedom' available at a given pressure and temperature - then the S is also large. Observations suggest that reactions are spontaneous in the direction where the system has more microstates) must be greater than 0. ΔS refers to the change in entropy (S), so if ΔS > 0, the reaction is spontaneous, thus increasing its 'disorder.' ΔH is also another change that occurs and it represents bond enthalpy/energy, i.e how much energy required to 'break' a bond (for simplicity's sake). This unit is given in kJ/mol (a unit of energy). If it takes a higher amount of energy to break a specific bond, ΔH increases and vice versa. This is important because we can calculate ΔG of a reaction by subtracting ΔH by (T; a given temperature the reaction takes place, times ΔS). The equation looks like this: ΔG = ΔH - TΔS. Ultimately, we can see that if ΔH is a large number (304.2 kJ/mol) and ΔS of a reaction is a given constant (these are measurements that can be found for different compounds on websites or textbooks. In this case, I've come up with a hypothetical number: 202.5 kJ/mol. Note: if we are just looking at entropy, this reaction should be spontaneous. but we must consider bond energy. Because it's a greater number, entropy is insignificant to the spontaneity of the reaction. Also, J must be converted to kJ to solve the equation. G is usually given in units of kJ, sometimes J), if T is a lower number such as 298 or 300K, then the reaction will not be spontaneous due to the large ΔH value. ΔG > 0, making the reaction non spontaneous thus 'favoring' the reactants. To make sure ΔG < 0, we must increase T. Increasing the T value will increase the value of T times ΔS. So, when subtracting ΔH by TΔS we get a negative number. Now, it is important to note that this cannot happen in living organisms. Humans for example, have a somewhat constant body temperature. The average human body is 37 degrees Celsius plus or minus 0.6. Although temperature can fluctuate a little bit, large fluctuations are dangerous to humans. A body temperature above 41 degrees Celsius can lead to organ failure and ultimately death. Because raising the temperature of the human body for non-spontaneous reactions is not the ideal method, the best way a non-spontaneous reaction can be spontaneous is the concept of coupled reactions. This is where the reaction of ATP breaking down into ADP or ATP hydrolysis comes into place.
 |
| Source: Hyper Physics |
Which Organisms Have ATP and What is its Function?
Almost all eukaryotes produce and maintain ATP. ATP is created in the mitochondria also known as the "powerhouse of the cell". Some parasite species are found to have ATP synthase, although the function of ATP isn't all that clear. Both bacteria and parasites can still produce ATP via glycosis since they do not have mitochondria. The ATP produced during glycosis is very small compared to the ATP created by eukaryotes such as humans, dogs, whales, etc. For parasites, the host provides oxygen and other nutrients and that's the main way parasites produce ATP. Parasites use the ATP for a source of energy, since the amount made from glycosis is very small. This continues until the host dies or the parasite is ejected from the system. Bacteria differs from parasites; they have a similar structure to mitochondria. Both bacteria and mitochondria produce ATP in a similar way, fuel from oxygen and glucose to create a phosphate bond to ADP. The main difference is that in mitochondria, the ATP production process occurs inside the organelle while in bacteria it occurs in the cell membrane.
The citric acid cycle is active in the inner membrane, this generates NADH which enters the electron transport chain on the inner membrane and pump protons (H+) into the mitochondrial intermembrane space. Those protons are pushed back into the inner membrane by ATP synthase. ATP synthase is an enzyme that is used to create ATP. The end product of the cycle is ATP which is stored in the mitochondria for later use.
The ATP process is similar in bacteria:
If simple prokaryotes and complex living organisms share the same molecule (ATP), it must be essential for life. I want to focus on ATP's specific functions in complex organisms and why ATP is an important part of certain processes. As mentioned earlier, the breaking of a phosphate bond in ATP releases a lot of energy, producing ADP. This reaction is coupled with many non-spontaneous reactions in the body to make them spontaneous. Thus, creating a product at a greater rate for the body to use.
Some of the reactions coupled with ATP:
- Conversion of acetic acid to acetyl CoA: ACSS2 in the process of ethanol breakdown
- Actin movement during muscle contraction/relaxation
- Sodium/Potassium pumps in nerve cells
Conversion of acetic acid to acetyl CoA: ACSS2
Ethanol is a toxic compound that can be found in a variety of things including fruit and alcohol. Ethanol occurs when yeast ferments sugars. This can happen naturally, i.e, in ripe or overly ripe fruit, or it can be found in alcohol. Due to adaptation and evolution, living organisms today can break down ethanol so that the volatile, flammable compound does not stay in the system to harm the organism's body. The breakdown of ethanol takes multiple steps. The step that ATP is involved in is the conversion of acetic acid to acyetyl CoA. This is a vital step in ethanol breakdown because it transports the carbon atoms from the acetyl group to the citric acid cycle (this cycle is mentioned during the ATP production process in mitochondria. The carbon atoms are oxidized and used for energy production). The enzyme involved in the transformation of acetic acid in acetyl CoA is ACSS2. This is a cytosolic enzyme that catalyzes the activation of acetate for lipid synthesis and energy generation. The protein acts as a monomer and produces from acetyl CoA. This reaction requires ATP. Once acetyl CoA is formed, its carbon atoms are used in the citric acid cycle. The breakdown of ethanol into the different steps not only removes the toxic compound out of the bloodstream/system, but is ultimately converted into energy used to produce and maintain ATP in the organism's body.Actin movement during muscle contraction/relaxation
Muscle contraction refers to the activation of tension-gathering sites within muscle fibers. This is when the muscle tension changes but the length remains the same, or the muscle length changes but the muscle tension remains the same. This process is what helps us humans and other vertebrates move. ATP plays an important part in this process by disconnecting actin from myosin. It is then hydrolyzed by the myosin molecule to produce the energy required for muscle contraction. This description of the different biochemical steps involved in muscle contraction is referred to as the Lymn-Taylor actomyosin ATPase hydrolysis mechanism. This process can be given in four steps:- The actin-myosin bridge very rapidly dissociates due to ATP binding to myosin (when a reaction is coupled, the spontaneous reaction will consume a specific reactant, causing more product).
- The free myosin bridge moves into position to attach to actin, during which ATP is hydrolyzed.
- The free myosin bridge along with its hydrolysis products rebinds to the actin strand.
- The cross-bridge generates force, and actin displaces the reaction products (ADP and Pi) from the myosin cross-bridge. This is the rate-limiting step of contraction.
Sodium/Potassium pumps in nerve cells
Sodium/potassium pumps also known as sodium/potassium -ATPase is an enzyme found in the plasma membrane of all animal cells, including humans. This enzyme pumps sodium out of the cell membrane while pumping potassium into the membrane. This process works against electronegativity and diffusion. Essentially, the positive ions want to neutralize the negative ions, balancing the charge. The sodium/potassium -ATPase helps regulate and maintain the resting potential of a nerve cell. This pump basically forces the more positive Na+ (sodium ions) out of the cell membrane, while bringing/keeping some K+ (potassium ions) in. This keeps the nerve cell at a slightly negative charge (its resting potential), so that it can get reactivated again once strong enough stimuli interact with the neuron. ATP can be compared to a gate: the sodium ions bind with ATP and the splitting of ATP (ATP hydrolysis: formation of ADP) exerts energy and is a spontaneous reaction. This ultimately changes the shape of the channel, releasing the sodium ions to the outside of the membrane. Now, two potassium ions bond with the channel, the pump pushes the potassium ions into the inside of the membrane also releasing a phosphate and ATP is remade. This process is constantly occurring in our bodies. Without ATP, the pump would not be able to work (since it requires a lot of energy) and our neurons would not be able to go back to its resting state.
 |
| ATP & Na+/K+ Pump (Source: Celtic Sea Salt) |
Recall the scenario at the beginning of the post: the reason 'you' feel energized after eating, is because your body has done this exact process (in simple terms): food to glucose, glucose oxidized to CO2 and H2O. Both this reaction and the ATP hydrolysis coupled reactions help produce energy for your body. Ultimately, keeping it running.
References & Other Links Relating to ATP/ATP Hydrolysis
'Free Energy' Gibbs and ATP
1) Better Biochemistry: The Free Energy of ATP Hydrolysis
2) Metabolism Is Composed of Many Coupled, Interconnecting Reactions
3) FREE ENERGY, ATP HYDROLYSIS PHOSPHORYLATION POTENTIAL
4) The Structure and Hydrolysis of ATP
5) ATP and reaction coupling
6) Catalysis and the Use of Energy by Cells
7) Professor Nauli's Webpage
Muscle Contraction
1) ATP Analogs and Muscle Contraction: Mechanics and Kinetics of Nucleoside Triphosphate Binding and Hydrolysis
2) Mechanical effects of muscle contraction increase intravascular ATP draining quiescent and active skeletal muscle in humans
3) Different effects of ATP on the contractile activity of mice diaphragmatic and skeletal muscles
4) A model for actin polymerization and the kinetic effects of ATP hydrolysis
Mitochondria, Bacteria/Parasites & ATP
1) ATP Synthase Complex of Plasmodium falciparum
2) Use of l-Proline and ATP Production by Trypanosoma cruzi Metacyclic Forms as Requirements for Host Cell Invasion
3) Reconstruction of Sugar Metabolic Pathways of Giardia lamblia
4) Mitochondria-Dependent Reactive Oxygen Species-Mediated Programmed Cell Death Induced by 3,3′-Diindolylmethane through Inhibition of F0F1-ATP Synthase in Unicellular Protozoan Parasite Leishmania donovani
5) Host Cell Egress and Invasion Induce Marked Relocations of Glycolytic Enzymes in Toxoplasma gondii Tachyzoites
6) Niche metabolism in parasitic protozoa
7) On the Origin of Mitochondria: Reasons for Skepticism on the Endosymbiotic Story
8) Mitochondrial Evolution
9) Reproduction, symbiosis, and the eukaryotic cell
10) On the Origin of the Eukaryotic Cell
Sodium/Potassium Pump
1) The Sodium-Potassium Pump
2) STRUCTURE AND MECHANISM OF Na,K-ATPASE

